Efficacy and Safety Data
In a clinical trial, FEIBA prophylaxis delivered a reduction in median Annual Bleed Rate (ABR) compared with on-demand treatment1
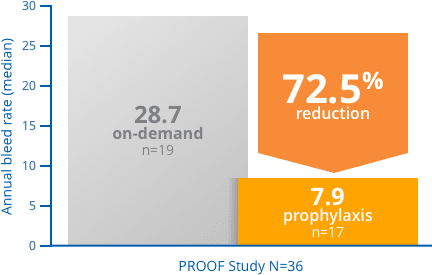
PROOF Study: a phase 3, prospective, randomized, 12-month, open-label study in 36 patients with hemophilia A or B and with inhibitors. Seventeen patients received FEIBA [Anti-Inhibitor Coagulant Complex] prophylactically (85 ± 15 U/kg every other day), while 19 patients received FEIBA on-demand for the resolution of acute bleeding episodes (dosages determined by treating physicians).3
Of those patients who achieved zero bleeding events, 2 out of 3, completed the study.3
FEIBA prophylaxis delivered a reduction in joint bleeds compared with on-demand treatment3
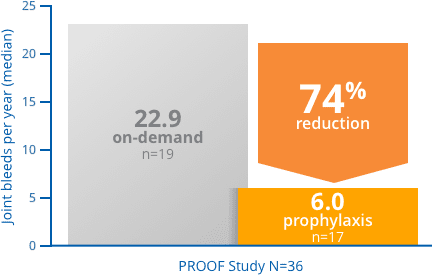
PROOF Study: a phase 3, prospective, randomized, 12-month, open-label study in 36 hemophilia A or B patients with inhibitors. Seventeen patients received FEIBA prophylactically (85 ± 15 U/kg every other day), while 19 patients received FEIBA on-demand for the resolution of acute bleeding episodes (dosages determined by treating physicians).3
*Seven new target joint bleeds in 5 subjects treated with prophylaxis compared with 23 new target joint bleeds in 11 subjects in the on-demand arm.1,† †Not significant
Target Joint Bleeds = Ankle, knee, elbow, or hip joint in which ≥4 bleeding episodes occurred in the same joint within a 6-month period during the study and was not a target joint bleeds at study initiation.
The adverse reactions in >5% of subjects reported in the randomized, prospective prophylaxis trial included anemia, diarrhea, nausea, vomiting, hepatitis B Surface Antibody Positive, and hemarthrosis.
Selected Important Risk Information for FEIBA [Anti-Inhibitor Coagulant Complex]
WARNING: EMBOLIC AND THROMBOTIC EVENTS
- Thromboembolic events have been reported during post-marketing surveillance following infusion of FEIBA, particularly following the administration of high doses (above 200 units per kg per day) and/or in patients with thrombotic risk factors.
- Monitor patients receiving FEIBA for signs and symptoms of thromboembolic events.
CONTRAINDICATIONS
FEIBA is contraindicated in patients with:
- History of anaphylactic or severe hypersensitivity reactions to FEIBA or any of its components, including factors of the kinin generating system
- Disseminated intravascular coagulation (DIC)
- Acute thrombosis or embolism (including myocardial infarction)
WARNINGS AND PRECAUTIONS
Thromboembolic events (including venous thrombosis, pulmonary embolism, myocardial infarction, and stroke) can occur, particularly following the administration of high doses (>200 units/kg/day) and/or in patients with thrombotic risk factors.
Offer your patients the opportunity to control unexpected bleeds1
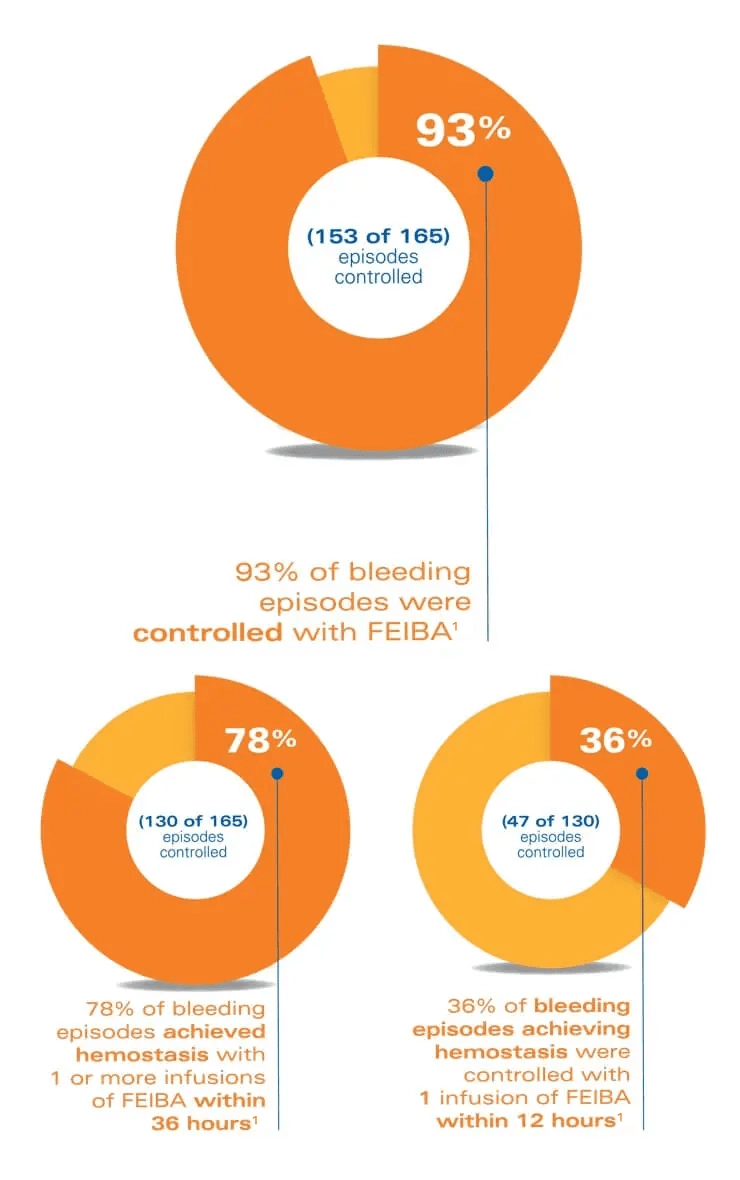
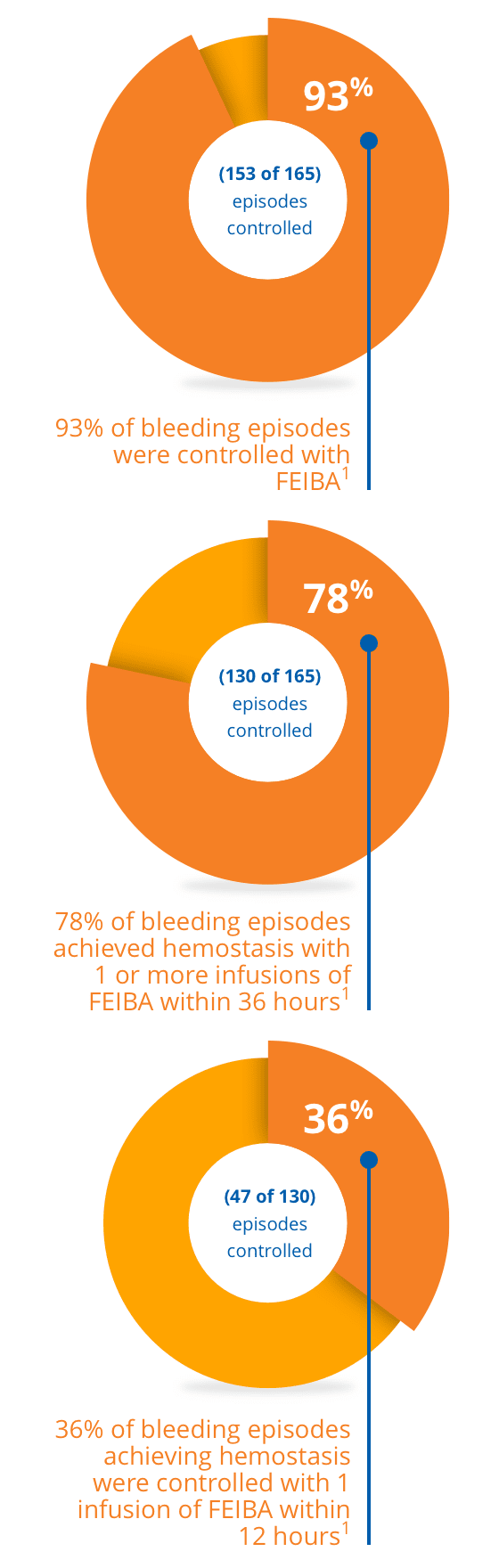
*Dose dependent on the location and extent of bleeding and the patient's clinical condition.
A multicenter, randomized, prospective trial was conducted in 44 hemophilia A subjects with inhibitors, 3 hemophilia B subjects with inhibitors, and 2 acquired factor VIII inhibitor subjects. The trial was designed to evaluate the efficacy of FEIBA in the treatment of joint, mucous membrane, musculocutaneous, and emergency bleeding episodes such as central nervous system hemorrhages and surgical bleedings.
The adverse reactions reported from two prospective clinical trials in which FEIBA was used for the treatment of acute bleeding episodes were chills, chest pain, chest discomfort, dizziness, dysgeusia, dyspnea, hypoesthesia, increase of inhibitor titer (anamnestic response), nausea, pyrexia, and somnolence. Specifically, the first trial was a multicenter randomized, double-blind trial in 15 hemophilia A subjects with inhibitors to factor VIII. The second trial was a multicenter FEIBA study conducted in 44 hemophilia A subjects with inhibitors, 3 hemophilia B subjects with inhibitors and 2 acquired factor VIII inhibitor subjects. Of the 489 infusions used to treat acute bleeds during the second trial, 18 (3.7%) caused minor transient reactions of chills, fever, nausea, dizziness and dysgeusia. Out of 49 subjects, 10 (20%) had a rise in their inhibitor titers after treatment with FEIBA. Five of these subjects (50%) had increases that were, tenfold or more, and 3 (30%) of these subjects received factor VIII or IX concentrates within 2 weeks prior to treatment with FEIBA. These anamnestic rises were not associated with decreased efficacy of FEIBA.
In a clinical trial, FEIBA was rated effective during surgical procedures5
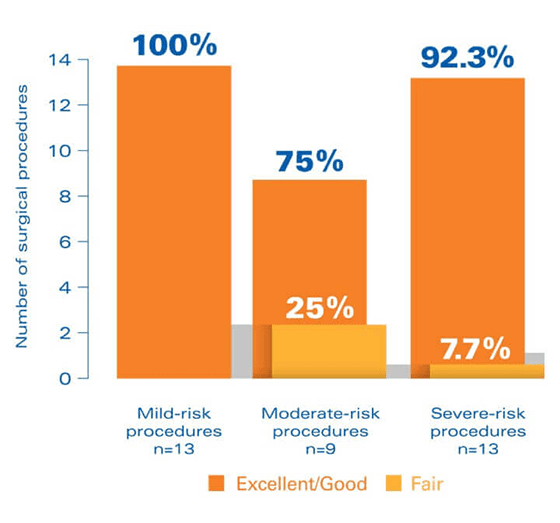
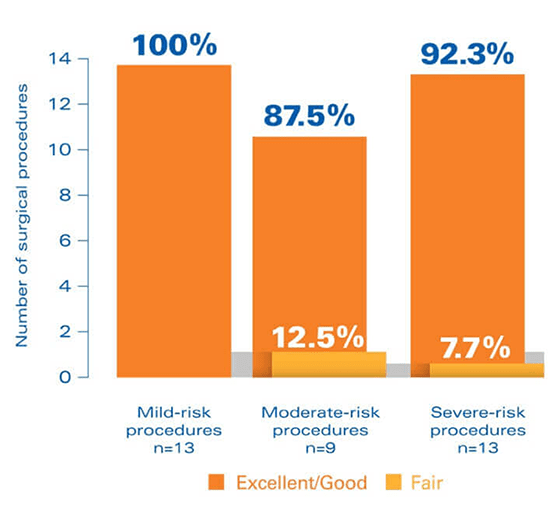
Study design: The SURgical interventions with FEIBA (SURF) study was an open-label, prospective, postauthorization study, specifically designed to clinically evaluate the perioperative use of FEIBA and accumulate a database of experience with perioperative FEIBA treatment that can be used to identify best practices in the surgical hemostatic management of hemophilia patients with inhibitors.This noninterventional, observational cohort study evaluated outcomes for 35 surgical procedures in 24 patients.
In the SURF study, hemostatic efficacy was defined as follows: Excellent = hemostatic expectations were met or exceeded in light of previous experience with bypassing agents. Good = efficacy was “somewhat less than expected” but still adequate compared with previous bypassing therapy. Fair = hemostasis was significantly less than expected compared with previous bypassing therapy.
There were 2 adverse events (AEs) attributed to FEIBA5
- Treatment–related, serious AE: 1 case of a clot in an arteriovenous fistula occurred during a moderate-risk surgery
- Not treatment–related, serious AE: 1 case of anemia and 1 case of hemarthrosis; each occurred during severe-risk surgeries
- Treatment–related, nonserious AE: 1 case of postoperative anemia
Selected Important Risk Information for FEIBA [Anti-Inhibitor Coagulant Complex]
WARNINGS AND PRECAUTIONS (continued)
Patients with DIC, advanced atherosclerotic disease, crush injury, septicemia, or concomitant treatment with recombinant factor VIIa have an increased risk of developing thrombotic events due to circulating tissue factor or predisposing coagulopathy. Potential benefit of treatment should be weighed against potential risk of these thromboembolic events.
Infusion should not exceed a single dose of 100 units/kg and daily doses of 200 units/kg. Maximum injection or infusion rate must not exceed 2 units/kg/minute. Monitor patients receiving >100 units/kg for the development of DIC, acute coronary ischemia and signs and symptoms of other thromboembolic events. If clinical signs or symptoms occur, such as chest pain or pressure, shortness of breath, altered consciousness, vision, or speech, limb or abdomen swelling and/or pain, discontinue FEIBA and initiate appropriate diagnostic and therapeutic measures.
Safety and efficacy of FEIBA for breakthrough bleeding in patients receiving emicizumab has not been established. Cases of thrombotic microangiopathy (TMA) were reported in a clinical trial where subjects received FEIBA as part of a treatment regimen for breakthrough bleeding following emicizumab treatment. Consider the benefits and risks with FEIBA if considered required for patients receiving emicizumab prophylaxis. If treatment with FEIBA is required for patients receiving emicizumab, the hemophilia treating physician should closely monitor for signs and symptoms of TMA. In FEIBA clinical studies TMA has not been reported.
Dosing Guidelines
The FEIBA Guidelines for Dosing is an educational guide for healthcare professionals only.
Learn MoreMechanism of Action
Multiple clotting factors of FEIBA achieve hemostasis in an in vitro analysis.2
See How It Works![FEIBA [Anti-Inhibitor Coagulant Complex]. Please see Detailed Important Risk Information, including BOXED WARNING on Embolic and Thrombotic events and full PI.](/us/content/dist/images/Hcp/logo.png)
