How It Helps
Here to help you control bleeds and manage your hemophilia A OR B with inhibitors
There are 2 ways you can take FEIBA. Depending on the treatment you and your doctor decide on, it can help prevent bleeds or help stop them.20
PROPHYLACTIC treatment
Routine treatment, also known as prophylaxis, means you take FEIBA regularly, before a bleed occurs.
On-demand
treatment
This means you take FEIBA as soon as possible after a bleed starts.
The Medical and Scientific Advisory Council (MASAC) of the National Hemophilia Foundation recommends the consideration of prophylactic treatment in patients with inhibitors.21,22
If you're living with inhibitors, you can help reduce bleeds with FEIBA routine prophylaxis.
FEIBA Prophylactic use: See how this option stacks up
In a clinical study, people on FEIBA routine prophylaxis experienced a significant reduction in the number of bleeds in one year, also known as the annual bleed rate (ABR), as compared with on-demand treatment.1,23*
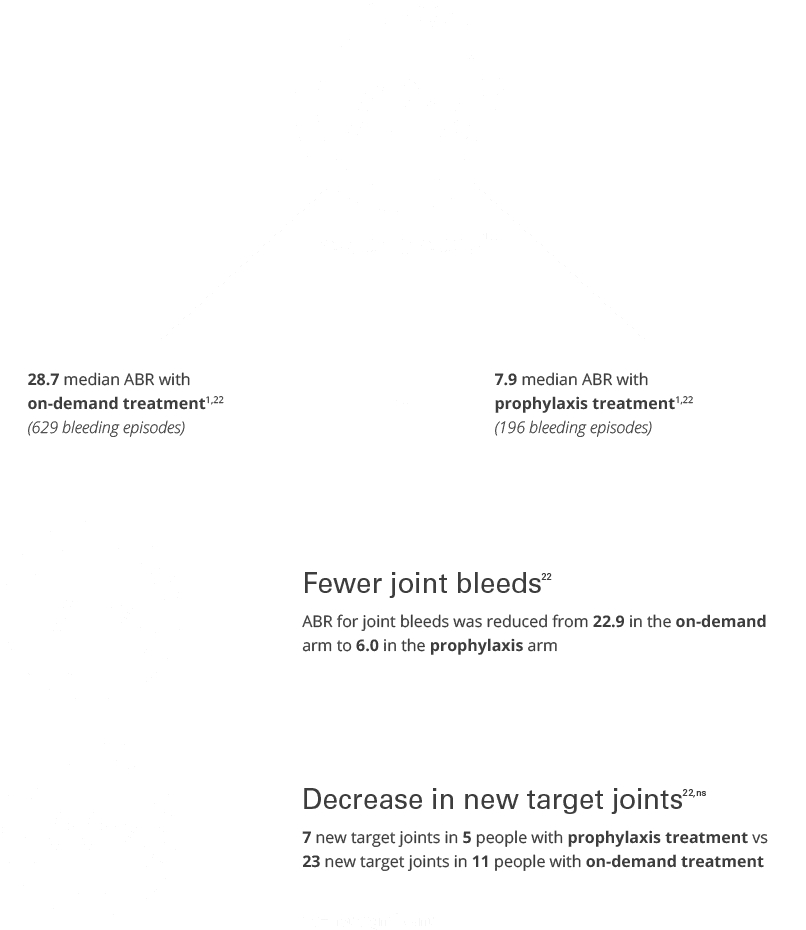


28.7 median ABR with
on-demand treatment1,23
(629 bleeding episodes)
vs
7.9 median ABR with
prophylactic treatment1,23
(196 bleeding episodes)
Fewer joint bleeds23
ABR for joint bleeds was reduced from 23 in the on-demand arm to 6 in the prophylaxis arm23
66% Fewer new target joints1,23,ns,†
7 new target joints in 5 people with prophylactic treatment vs 23 new target joints in 11 people with on-demand treatment
ns=not significant
*Based on results from the FEIBA PROOF clinical study of 36 hemophilia A and B patients with inhibitors receiving FEIBA for prophylaxis or on-demand treatment for 12 months.1,23
†Target joint—ankles, knees, elbows, and/or hips with at least 4 bleeds over 6 months.
Selected Important Risk Information for FEIBA [Anti-Inhibitor Coagulant Complex]
WARNING: EVENTS INVOLVING CLOTS THAT BLOCK BLOOD VESSELS
- Blood clots that block blood vessels and their effects have been reported during post-marketing surveillance following infusion of FEIBA, particularly following administration of high doses (above 200 units per kg per day) and/or in patients at risk for forming blood clots.
- If you experience any of these side effects, call your doctor right away.
Who should not use FEIBA?
You should not use FEIBA if:
- You had a previous severe allergic reaction to the product
- You have Disseminated Intravascular Coagulation (DIC), or signs of small blood vessel clots throughout the body
- You have sudden blood vessel clots or blocked blood vessels, (such as, heart attack or stroke)
Annual bleed rate with FEIBA Prophylaxis
When the annual bleed rate (ABR) results from the same study were grouped by bleeding cause and/or type, FEIBA prophylaxis treatment showed significant differences vs on-demand treatment.23*
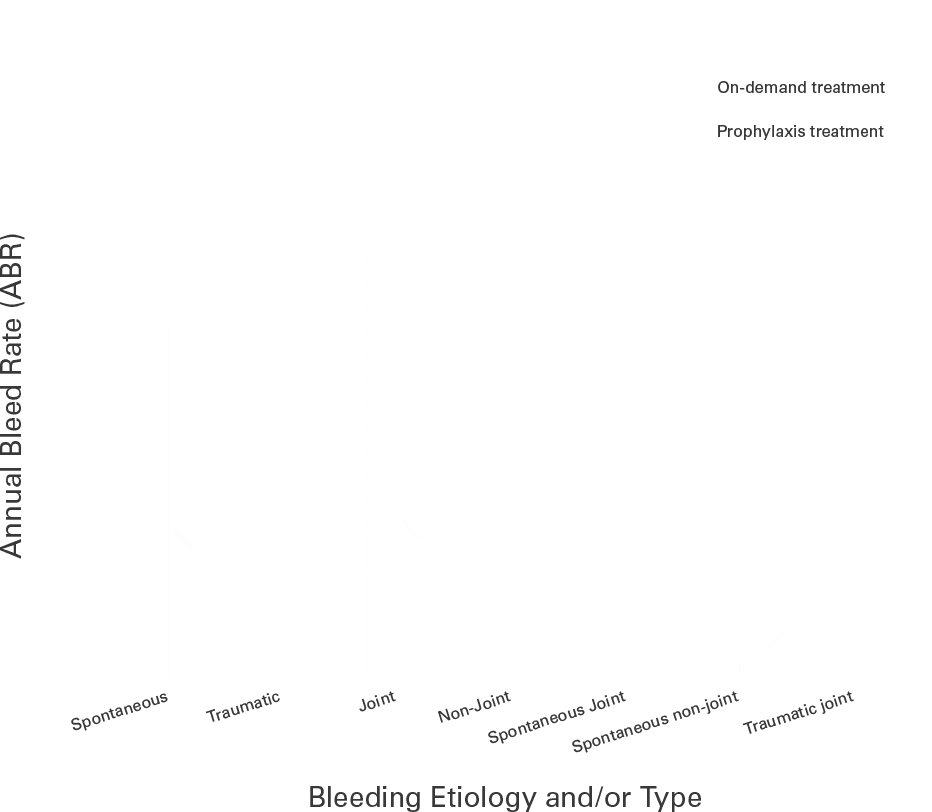
spontaneous
bleeds23
FEIBA prophylaxis significantly reduced ABR for all bleeds, compared with on-demand treatment.23
*Based on results from the FEIBA PROOF clinical study of 36 hemophilia A and B patients with inhibitors receiving FEIBA for prophylaxis or on-demand treatment for 12 months.1
Selected Important Risk Information for FEIBA [Anti-Inhibitor Coagulant Complex] (Continued)
What other important information should I know about FEIBA?
Events involving blood clots blocking blood vessels (such as blood clot in vein, blood clot in the lung, heart attack, and stroke) can occur with FEIBA, particularly after receiving high doses (above 200 units per kg per day) and/or in patients with risk factors for clotting.
At first sign or symptom of a sudden blood vessel clot or blocked blood vessel (such as chest pain or pressure, shortness of breath, fever, altered consciousness, vision, or speech, limb or abdomen swelling and/or pain), stop FEIBA administration right away and seek immediate emergency medical treatment.
Infusion of FEIBA should not exceed a single dose of 100 units per kg body weight and daily doses of 200 units per kg of body weight. Maximum injection or infusion rate must not exceed 2 units per kg of body weight per minute.
The safety and efficacy of FEIBA for breakthrough bleeding in patients receiving emicizumab has not been established. Events of thrombotic microangiopathy (TMA), a condition where blood clots and damage occur in small blood vessels, were reported in an emicizumab (Hemlibra®) clinical trial where patients received FEIBA with emicizumab as part of a treatment plan for breakthrough bleeding. If you are on emicizumab and are taking or anticipate taking FEIBA for a breakthrough bleeding episode, tell your doctor immediately because there are specific safety considerations and you must be closely monitored by your hemophilia treater or treatment center.
Allergic reactions, including severe, sometimes fatal allergic reactions that can involve the whole body, can occur following the infusion of FEIBA. Stop using FEIBA promptly and call your doctor or get emergency treatment right away if you get a rash, hives or welts, experience itching, tightness of the throat, vomiting, abdominal pain, chest pain or tightness, difficulty breathing, lightheadedness, dizziness, nausea or fainting.
Because FEIBA is made from human plasma it may carry a risk of transmitting infectious agents, such as viruses, variant Creutzfeldt-Jakob disease (vCJD) and, theoretically, the Creutzfeldt-Jakob disease (CJD).
FEIBA on demand: another treatment option.
Bleeds can happen. If you don't infuse regularly with FEIBA, you can take FEIBA on demand to help stop bleeding episodes when they occur.
In clinical trials, FEIBA was effective at treating various types of bleeding episodes, including spontaneous and traumatic bleeds.
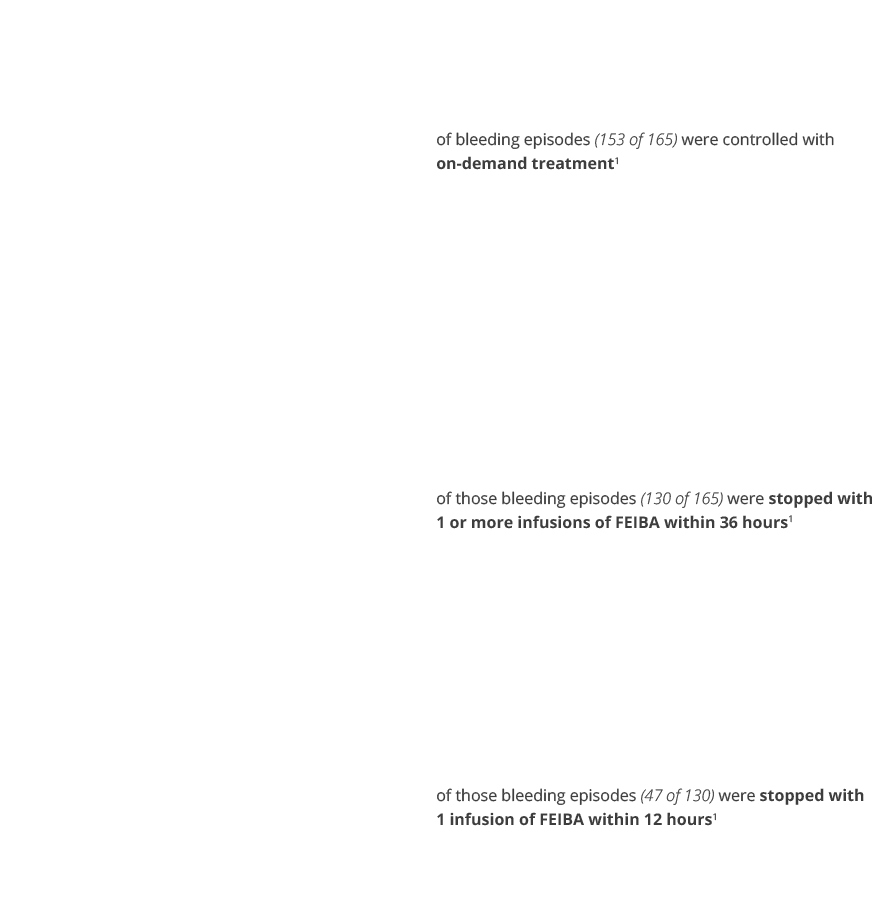
In a separate clinical study, FEIBA was used to treat 165 bleeding episodes and showed the following results1:
Up to 12 hours between doses reduces need for more frequent redosing1*
*Depends on bleed type. Some bleeds may require dosing at 6-hour intervals. Please consult with your doctor.
|
-0%1%2%3%4%5%6%7%8%9%10%11%12%13%14%15%16%17%18%19%20%21%22%23%24%25%26%27%28%29%30%31%32%33%34%35%36%37%38%39%40%41%42%43%44%45%46%47%48%49%50%51%52%53%54%55%56%57%58%59%60%61%62%63%64%65%66%67%68%69%70%71%72%73%74%75%76%77%78%79%80%81%82%83%84%85%86%87%88%89%90%91%92%93%94%95%96%97%98%99%100%
|
of bleeding episodes (153 of 165) were controlled with on-demand treatment1 |
|
-0%1%2%3%4%5%6%7%8%9%10%11%12%13%14%15%16%17%18%19%20%21%22%23%24%25%26%27%28%29%30%31%32%33%34%35%36%37%38%39%40%41%42%43%44%45%46%47%48%49%50%51%52%53%54%55%56%57%58%59%60%61%62%63%64%65%66%67%68%69%70%71%72%73%74%75%76%77%78%79%80%81%82%83%84%85%86%87%88%89%90%91%92%93%94%95%96%97%98%99%100%
|
of those bleeding episodes (130 of 165) were stopped with 1 or more infusions of FEIBA within 36 hours1 |
|
-0%1%2%3%4%5%6%7%8%9%10%11%12%13%14%15%16%17%18%19%20%21%22%23%24%25%26%27%28%29%30%31%32%33%34%35%36%37%38%39%40%41%42%43%44%45%46%47%48%49%50%51%52%53%54%55%56%57%58%59%60%61%62%63%64%65%66%67%68%69%70%71%72%73%74%75%76%77%78%79%80%81%82%83%84%85%86%87%88%89%90%91%92%93%94%95%96%97%98%99%100%
|
of those bleeding episodes (47 of 130) were stopped with 1 infusion of FEIBA within 12 hours1 |
FEIBA was previously licensed in the United States as FEIBA VH and FEIBA NF, and is now available as FEIBA. FEIBA and FEIBA NF are identical in formulation to FEIBA VH. Biochemical and preclinical studies have confirmed the comparability of FEIBA NF and FEIBA VH.
Selected Important Risk Information for FEIBA [Anti-Inhibitor Coagulant Complex] (Continued)
What are the possible side effects of FEIBA?
The most common side effects observed during the prophylaxis clinical study were low number of red blood cells, diarrhea, bleeding into a joint, positive test for hepatitis B surface antibodies, nausea, and vomiting.
The serious side effects seen with FEIBA are allergic reactions and clotting events involving blockage of blood vessels, which include stroke, blockage of the main blood vessel to the lung, and deep vein blood clots.
Call your doctor right away about any side effects that bother you during or after you stop taking FEIBA.
What other medications might interact with FEIBA?
Talk with your doctor about the possibility of formation of blood clots when taking drugs that may prevent clot breakdown such as tranexamic acid, and aminocaproic acid. There have not been adequate studies of the use of FEIBA and rFVIIa (NovoSeven®), or emicizumab together, or one after the other. Use of drugs that may prevent clot breakdown within approximately 6 to 12 hours after the administration of FEIBA is not recommended.
Want to learn more? Check out our About FEIBA page.
![FEIBA [Anti-Inhibitor Coagulant Complex]. Please see Detailed Important Risk Information, including BOXED WARNING on embolic and thrombotic events and Full PI.](/us/content/dist/images/Patient/logo.png)
