About FEIBA
Inhibitors are tricky. We can help.
FEIBA is made to help you control bleeds and manage your hemophilia A or B with inhibitors to factors VIII and IX. It's also made with your safety in mind.1
40
YEARS
We have a history of helping people with inhibitors. FEIBA has been approved for on-demand treatment for over 40 years.14
78
COUNTRIES
FEIBA is approved in more than 70 countries worldwide.15
60
COUNTRIES
FEIBA has been indicated for prophylaxis in more than 60 countries.15
Developed with HIGH standards
You need to know that what you put into your body is as safe as possible. The purification process for FEIBA was created with your safety in mind.1
FEIBA is made with care at every step, from the plasma sourced and screened to the purification and testing.1
Made with care every step of the way1,16
All plasma donors pass rigorous screenings for qualification
Plasma from qualified donors is held for 60 days
Each plasma donation is tested for human immunodeficiency virus (HIV), hepatitis B virus (HBV), and hepatitis C virus (HCV)
Next up: viral serologic testing for HBV surface antigen, HCV antibody, HIV-1 and HIV-2 antibodies, and HIV p24 antigen is performed on all plasma donations
And more: nucleic acid amplification tests (NAT) for HIV, HCV, HBV, hepatitis A virus (HAV), and parvovirus B19 (PV B19)
FEIBA [Anti-Inhibitor Coagulant Complex] Purification Process1,16


As with all plasma-derived products, the risk of transmission of infectious agents cannot be totally eliminated. As of August 2020 (from self-reported databases), there have been no confirmed reports of transmission of hepatitis A, B, or C or human immunodeficiency virus (HIV) associated with the use of FEIBA since the introduction of vapor heat treatment (ongoing surveillance indicates this remains valid).17
Selected Important Risk Information for FEIBA [Anti-Inhibitor Coagulant Complex]
WARNING: EVENTS INVOLVING CLOTS THAT BLOCK BLOOD VESSELS
- Blood clots that block blood vessels and their effects have been reported during post-marketing surveillance following infusion of FEIBA, particularly following administration of high doses (above 200 units per kg per day) and/or in patients at risk for forming blood clots.
- If you experience any of these side effects, call your doctor right away.
Who should not use FEIBA?
You should not use FEIBA if:
- You had a previous severe allergic reaction to the product
- You have Disseminated Intravascular Coagulation (DIC), or signs of small blood vessel clots throughout the body
- You have sudden blood vessel clots or blocked blood vessels, (such as, heart attack or stroke)
Here's how FEIBA works
Bypassing agents—like FEIBA—are important treatments for people with inhibitors. Because factor VIII or IX treatment will no longer be effective, FEIBA gets around the need for these factors and helps your blood form clots to stop bleeding.1,18,19
In vitro analysis
FEIBA contains several different clotting factors—factors II, IX, and X, mainly non-activated; and factor VII, mainly in the activated form.1
FEIBA acts at multiple sites in the clotting cascade.19
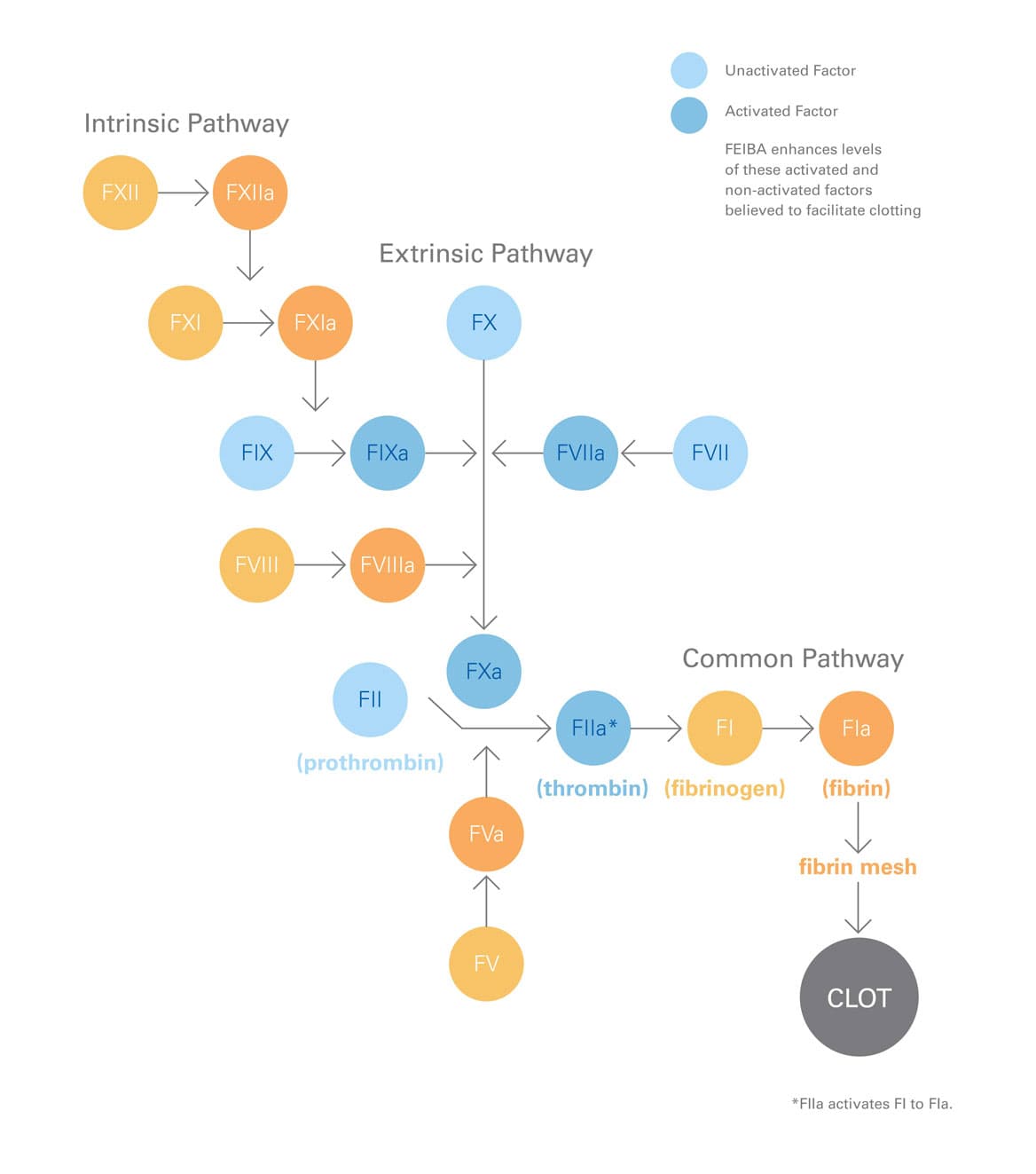
FEIBA acts at multiple sites in the clotting cascade and helps restore thrombin generation in people who have hemophilia with inhibitors. Thrombin helps with the formation of the fibrin clot, which helps stop bleeding.1,19
Bypassing inhibitors: the play-by-play
- For hemophilia A and B patients with inhibitors, clotting doesn't occur naturally during bleeding due to missing factor VIII or factor IX.3
- As shown above, FEIBA bypasses (or "gets around") the need for these factors and helps your blood form clots to stop bleeding.1,19
- FEIBA provides several different clotting factors—mainly unactivated factors II, IX, and X; and mainly activated factor VII—and works at different sites in the clotting system.1,19
- This restores thrombin generation and helps with the formation of the fibrin clot, which stops bleeding.1,19
FREEDOM OF CHOICE™
Find out if you're eligible for 15 free trial doses of FEIBA by completing the form with your healthcare provider.
Download the formWant to learn more? Check out our How It Helps page.
![FEIBA [Anti-Inhibitor Coagulant Complex]. Please see Detailed Important Risk Information, including BOXED WARNING on embolic and thrombotic events and full PI.](/us/content/dist/images/Patient/logo.png)



